Introduction:
Gold nanocolloids are particularly useful in optical absorption/scattering applications due to their strong optical responses and their biocompatible nature. The combination of the unique optical and biological properties of gold nanocolloids makes them excellent candidates for biomedical applications, either as contrast enhancers for optical imaging or nanocarriers for drug delivery, or both. Gold nanoparticles have exceptionally strong absorption in the visible and NIR spectral range which is critical for optical and photoacoustic imaging. Gold nanoparticles have been proven to produce photoacoustic signals almost an order of magnitude higher than organic dyes in solutions of equal absorbance. In comparison with organic dyes, gold nanoparticles are more stable and are free from photobleaching, which is critical for long-term monitoring. Gold nanocolloids can be engineered into different shapes, such as nanospheres, nanoshells, nanorods, and nanocages, each with different physical properties including optical absorption spectra.
In our study on animal models, the excellent sensitivity of photoacoustic imaging (PAI) to gold nanoparticles, including both gold nanorods and gold nanowonton, has been demonstrated. A novel technique for monitoring the in vivo behaviors of gold particles (AuNPs) using γ-imaging has been developed. AuNPs were radiolabeled using 125I sodium iodide in a simple and fast manner with high yield and without disturbing optical properties. Radiolabeled AuNPs were successfully visualized by radioisotope tag, allowing longitudinal in vivo studies to be performed repeatedly in the same animal.
Example results:
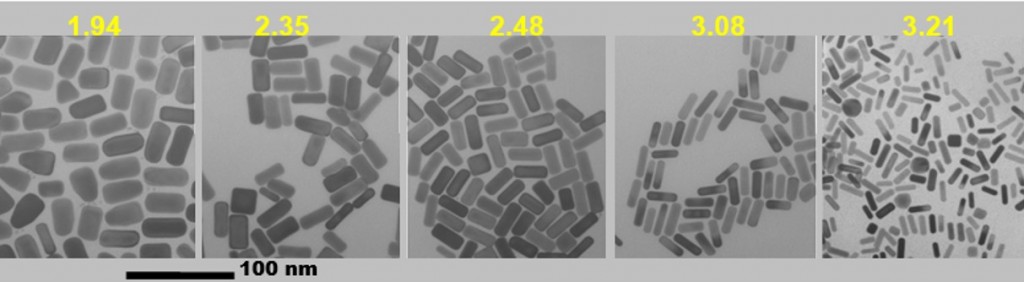
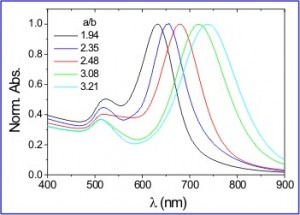
Image Courtesy of Dr. L. Liz-Marzan.
Tuning the absorption peak of gold nanorods by varying their aspect ratio.
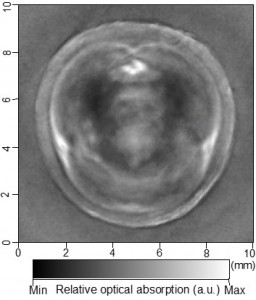
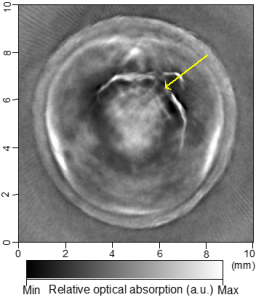
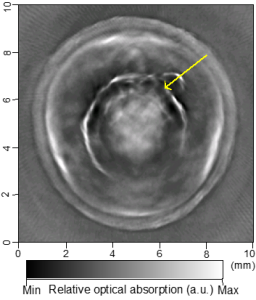
Cross-sectional photoacoustic tomography (PAT) of a rat tail joint enhanced by gold nanorod contrast agent. (A) Image taken before administration of the nanorods locally into the joint space. (B) Image taken after the first injection of 100 attomole nanorods. (C) Image taken after the second injection of 100 attomole nanorods. The arrows indicate the route of nanorod administration. The distribution of gold nanorods in the joint space can be recognized clearly.
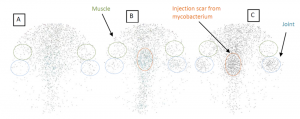
By conjugating gold nanorods (AuNRs) with ICAM-1 antibodies and also radiolabeling them with 125I, specifically targeted delivery of gold nanorods to the inflammatory articular tissues has been achieved in an experiment on an arthritis rat model. The above shows the Gamma camera images each covering the rear part of a rat body, where the blue circles mark the inflammatory ankle joints, and green circles mark the muscle areas for comparison. As a control, (A) shows the image of a normal rat injected with 125I-ICAM-GdNRs (targeting nanoparticles). As another control, (B) shows the image of an arthritis rats injected with 125I-GdNRs (non-targeting nanoparticles). (C) shows the image of an arthritis rats injected with 125I-ICAM-GdNRs.